How to write a superb Cost Effective Validation
The title - How to write a superb Cost Effective Validation Schedule or plan, has already been highlighted by the FDA, WHO and the EC. All have repeatedly warned that intensity of regulatory compliance inspections was likely to increase in the regulatory drive to ensure that controlled product has been produced in compliance with current Good Manufacturing Practices (cGMP) and meets or exceeds all required specifications; as listed in the User Requirements Specification (URS).
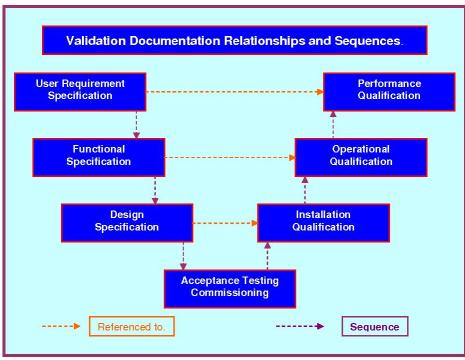
The evolution of Validation required the development of suitable documents, such as the VMP, URS, DQ, IO, OQ, P1Q, P2Q. Many manufacturing processes produce a product or part of a product that cannot be tested without damaging it, or indeed destroying it. The introduction of QA allowed manufacturers to build and destructively test, then using rigorously enforced quality controls replicate the tested product exactly, now knowing its performance and limitations.
Pharmaceutical, biopharm and medical device companies must demonstrate to the regulators that their whole operation is under similar control. Quality Control that will ensure all operations are documented, all materials are verified as correct and all equipment is qualified as fit for purpose. All this must be in place and operational prior to receiving regulatory approval to manufacture. This status must be maintained to a standard that ensures consistent replication of all the production processes and without ever compromising the quality of that product. The maintenance of these standards is called Current Good Manufacturing Practice (cGMP). Being conversant wit cGMP enable one to understand the complexities of how to write a superb cost effective validation plan or schedule. This qualified or approved state is subject to continuous ministering and inspection by the regulatory authorities involved, as long as the product is in production.
GO TO DOCUMENT STORE AND VIEW NOW
Basic Validation Requirements.
cGMP 211.100.
Understanding that there must be written procedures for production and process control, laying out how to write a superb cost effective validation schedule designed to assure that the drug products have the identity, strength, quality, and purity they purport or are represented to possess. Such procedures shall include all requirements in this sub-part. These written procedures, including any changes, shall be drafted, reviewed, and approved by the appropriate organizational units and reviewed and approved by the quality control unit.
cGMP 820.70
Each manufacturer shall develop, conduct, control, and monitor production processes to ensure that a device conforms to its specifications. Where deviations from device specifications could occur as a result of the manufacturing process, the manufacturer must establish how to write a superb cost effective validation schedule of applicable process control procedures; that will be applied to all such indigenous processes to ensure conformance to specifications. Where process controls are needed they shall include:
(1) Documented instructions, standard operating procedures (SOP's).
(2) Monitoring and control of process parameters.
(3) Compliance with specified reference standards or codes.
(4) The approval of processes and process equipment.
(5) Criteria for quality of workmanship.
21 Years of retailing cGMP - 35k templates supplied to the industry.
12000002 VrrP Equip iss-4. $298.00
This Validation, Risk & Requirements Plan (VrrP) is one document designed specifically to replace three. The contents of the three original documents were completely revised and edited into a more compact and interactive format. This new format will make a very significant difference to the man hours required to produce and execute these documents. There will also be a very noticeable reduction in the time required for the reviewing and approving tasks. This new document titled the VrrP replaces the VP, VRA & URS and now compliments our equally new 4Q Protocol, which integrates the DQ/IQ/OQ/PQ into one document. This is an essential step forward for companies seeking to reduce validation costs without infringing regulatory standards.
12000006 4Q Equip iss-4. $298.00
4Q Equipment Validation Protocol (4Q-Equip) has been designed specifically to replace four standard protocols. By taking the contents of the four protocol and carefully weaving them into one notably easy to use protocol, we have made a significant advance in the task of streamlining validation documentation by reducing protocol numbers by close to 75%. The new bang up to date 4Q protocol replaces the DQ, IQ, OQ & PQ and now compliments our equally new VrrP Protocol. By integrating the old style DQ/IQ/OQ/PQ into one 4Q document there will be enormous savings in man hours in the authoring, reviewing, updating and approving tasks. For everyone's ease of editing; it is still written in word.
12000008 4Q Equip Package $499.00
This quite revolutionary two document package is all that is required to fully validate; to cGMP standards, equipment used in a regulated facility. A lot of effort has gone into ensuring that repetitive instructions and actions have been designed out and innovative and intuitive risk-based methodologies have been incorporated. Both documents are prefaced with a methods Standard Operating Practice (SOP) document. These SOP’s lead you through the task of converting these highly detailed templates into your very own company bespoke protocols. The hyperlinks and cross-references within the package are; not only unique, but also highly cost effective and intuitive to use. Each document is preloaded with the test scripts (complete with acceptance criteria). All test and inspection scripts are written in MS word, to facilitate simple editing of text, layout, tables and schematics.