SOP for cGMP REVIEW.
SOP for cGMP Review Scope.
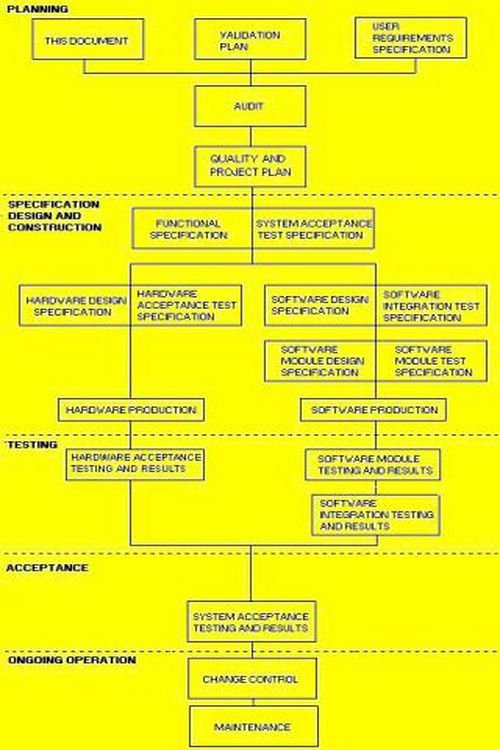
This SOP for cGMP Review, has been produced in response to numerous requests for such a document. Now you can do your own walk validation project assessment. The purpose of this procedure is to ensure that all relevant aspects of the proposed design are reviewed for cGMP compliance by the qualified personnel, in a controlled manner. Thus ensuring that the design and/or the facility meets the cGMP requirements, and that documentation is produced which demonstrates that the design conforms to the requirements of cGMP.
This procedure defines how cGMP Reviews are carried out for Engineering and/or Consultancy Projects from project kick-off through to handover, to ensure that the design intent has been carried out and the facility as designed (and subsequently as constructed) conforms to the requirements of cGMP.
PLEASE CLICK HERE TO GO TO SHOP AND PURCHASE THIS DOCUMENT
Requirements.
For pharmaceutical/biopharm facilities where it is necessary to seek and gain approval from Regulatory Authorities, it is essential to demonstrate that the design and/or facility complies with current Good Manufacturing Practice (cGMP) requirements as defined by the Regulatory Authorities from which approval is being sought.
The cGMP Review is undertaken to ensure that a design and/or facility conforms to the cGMP requirements and is fit for purpose. The requirement for Regulatory Compliance will be established during the proposal preparation having influence on the quality or efficiency of the product.
- PURPOSE
- SCOPE
- DEFINITIONS.
- METHOD
- Introduction.
- Timing.
- Information Requirements.
- Responsibilities.
- cGMP Review Team
- cGMP Review Meeting.
- Change Control.
- Follow Up / Close Out.
Qualification Documentation.
News for Today