How to write a superb Equipment Qualification.
Understanding how to write a superb Equipment Qualification protocol takes time and study. This can be greatly condensed by using a quality template. Such as Equipment Qualification ie; Installation Qualification (IQ), Operational Qualification (OQ), Performance Qualification (P1Q) and Equipment Qualification (P2Q), there is a degree of flexibility as regards to the content of these documents. Although the document title would appear to define their scope, it is possible and permissible to arrange some elements of testing in the most expedient sequence. I.e. do you wait for power 'on' to do IQ, perhaps? Perhaps not? It can be argued that when power is on you are operating the equipment (so power on testing should be in the OQ). Can IQ therefore be completed prior to power up?
Equipment Qualification Derivation.
VP - URS - DQ - VRA - IQ - OQ - P1Q - P2Q
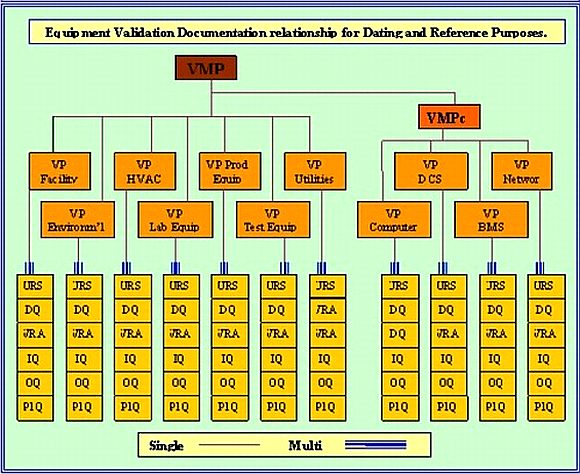
The VMP is not mandated by the FDA, but is always asked for in regulatory reviews and inspections. (URS - DQ - VRA) are mandated and self explanatory.
It can, and often is, hotly debated whether all the testing carried out in the FAT, SAT and commissioning stages, must be repeated in the equipment qualification. (i.e. are you going to repeat some of the testing four times?) This is where the VP becomes a vital document. Senior persons with a good understanding of the overall project, can decide the content and scope of each of these documents, obviously within the framework of existing cGMP legislation and guidelines. They can actually develop a quite unique sequence of equipment qualification tasks for a number of reasons (to reduce repetitive testing - to ease access problems – to sequence with other activities, and on), providing that their rationale is documented in the VP and posts a plausible justification. This is where understanding how to write a superb equipment qualification protocols becomes rather essential.
The VP is then used by the protocol writers as the official mandate for protocol content.
Question time?
What is Validation in the Pharmaceutical Industry
The planned verification of a group of facts by-way-of tangible evidence.
What does IQ OQ PQ Stand for?
Installation Qualification. Operational Qualification.
Performance Qualification.
What is OQ Test?
The OQ is a whole series of tests used to verify that item equipment qualification possess all the functional requirements detailed in the URS.
What is URS document?
User Requirements Specification. Level 1 - End users requirements. Level 2 - Support structure for Level 1 (Calibration, Maintenance, Training, Utilities. Level - 3 Software traceability.
Understanding how to write a Superb Equipment Qualification protocol.
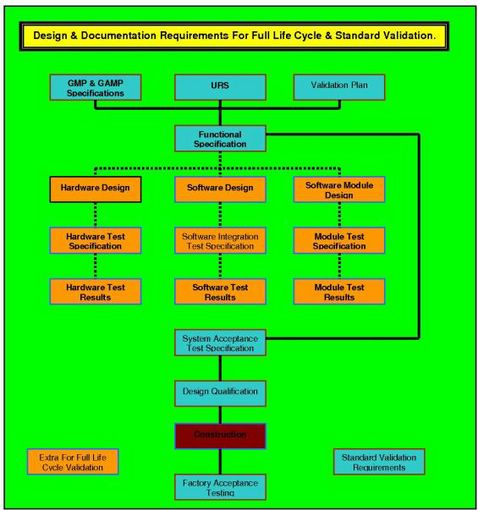
In the diagram below it can readily be seen that the URS, along with the regulatory requirements as detailed in GAMP 5, and the details of validation requirements as given in the VMP, come together to form a package from which the vendor can develop a fully legal, compliant and validateable product.
Equipment qualification templates are available for the activities shown grouped in the two colours (orange and blue). The blue color is the requirement for standard computer validation, while the orange color depicts the additional equipment qualification templates that are required when the process executed by the during equipment validation has been identified as critical.
The FDA regulators have decreed that software used in a manner that can affect the quality of the product, without leaving visible evidence, that damaged has occurred, must be deemed as critical to the quality of that product.
The regulators have further mandated that all such software be identified as such - and subjected; from concept to actual use, to Full Life Cycle Validation (FLCV) requirements. This additional level of validation requires the addition of further validation GMP equipment qualification templates as portrayed in the diagram in orange.
The vendor therefore (be they in, or out, of house), must produce a Quality Plan that ensures the software development will follow a validation evolution similar to that shown in the diagram. Your actual diagram must be planned and justified for the software system that you are proposing.
It is of paramount importance that your decision on software criticality is documented and that your Quality Plan (QP) is reviewed, approved by QA, the client & their representative; all prior to initiation of the design stage. Since these documents all form part of the equipment qualification-evaluation stage, you must expect regulatory inspectors to be interested in reviewing the content and the format of them.
Equipment Qualification Requirements.
Compliant Documentation.
All regulatory mandated protocols such as this Equipment Qualification protocol must be carefully prepared, with statements worded to preclude ambiguous and incorrect interpretations of the purpose of the statement. In addition there are quite separate requirements stipulated by the regulatory authorities. Documents presented to regulators during inspections that fail to satisfy these requirements, indicate to the regulator a lack of care and commitment on the part of the company. The exact opposite to what is required.
The FDA and regulatory authorities in general, are charged with increasing the level of compliance throughout the industry. This call for increased regulatory compliance, is driven by evidence that the industry is failing to match the standards set by other regulated industries.
Validation Online, have designed unique interactive documents for all validation activities such as VMP, URS, IQ, OQ, PQ, and many others. Understanding how to write a superb Equipment Qualification protocol takes time, however at times the clever way forward it to use good quality templates. These documents have all been designed to be cGMP, compliant, along with incorporating the structures the regulators look for. These documents are realistically priced to ensure it is much more cost effective for companies to download documents as they require them, than individually author them.
Tabletizing of Validation Results
Generally there is only one Validation Master Plan (VMP); although a case can be made for using two, if there is sufficient equipment to justify it. The Validation Plan (VP); on the other hand, is designed to collect together identical equipment. Although the IQ / OQ / PQ protocol tests and inspection would be only be written once; the execution results, would be tableted.
EQUIPMENT QUALIFICATION TEMPLATES.
21 Years of retailing cGMP - 35k templates supplied to the industry.
Equipment Validation Pack 2. $520.00
You want to validate a process line or new process equipment or individual process assembly stage equipment or just similar equipment that requires equipment qualification after having been subjected to major modification.
In this case you require the following validation protocols
VP, URS, VRA, DQ, IQ, OQ, PQ.
Level 2 Package consists of one of each of these.
User Requirements Specification (Issue 8) -- $115.00
This document was designed to be used as a live document up until the DQ is completed and approved. It uses three levels of URS, URS Level 1, 2 and 3, and is the only URS to guarantee traceability from the URS through to the final PQ and OQ functionality testing. A mandatory requirement for Full Life Cycle Validation of computer systems that are the subject of predicate rules. It can be used on mechanical, electrical Equipment qualification and software controlled, monitored or driven systems.
Validation Risk Assessment (Issue 11) -- $125.00
This is a robust and simple to execute document, one that will lead you through the process and deliver a result that can be used as the foundation for your validation activities. The VRA now includes the assessment table for categorizing and documenting the new 21 CFR Part 11 guidance ruling on what predicate data must be stored in a Part compliant system, along with the new broadsheet to establish your new database of part 11 records & equipment qualification.
The Installation Qualification (IQ) execution; verifies that the equipment, and its ancillary systems or sub-systems have been installed in accordance with installation drawings and or specifications. It further details a list of all the cGMP requirements that are applicable to this particular equipment qualification. These requirements must all be satisfied before the IQ can be completed and the Equipment qualification process is allowed to progress to the execution of the (OQ).
Operational Qualification, (Issue 10.) -- $115.00
You will find the step by step attached SOP delightfully simple and straightforward to use, as it takes you through the process of customization of your Operational Equipment Qualification Protocol template. Following the attached SOP will quickly and smoothly convert your template into an equipment Qualification Protocol. The OQ template comes complete with all the standard test scripts, more specialist test scripts can be found listed below. These can easily be pasted into the standard OQ, allowing you to quickly build your own fully detailed and referenced company bespoke Operational Equipment Qualification Protocol. Template for VMP.