PHARMACEUTICAL EQUIPMENT VALIDATION.
Pharmaceutical Equipment Validation Introduction.
Pharmaceutical Equipment validation or qualification to FDA cGMP standards, can be quite simple to achieve providing the
procurement stage has been thoroughly investigated and concisely
documented in accordance with a company approved process. The procurement process normally
starts with the production of a documented requirement or group of requirements
(URS).
For new builds this must be incorporated into the originating validation or
project plan (VP).
For existing facilities this should take the form of a CHANGE REQUEST (CR). As soon as management has agreed to proceed with the CR, approval should be issued to produce a VP. This plan must be all encompassing. It must give assurance that all aspects of the proposed CR have been studied and the CR impact on existing facilities, utilities, product and personnel have been defined and the appropriate corrective or support actions planned for. A fully detailed User Requirements Specification (URS) can now be authored reviewed and published. Since developing the URS may raise problems that could not be anticipated when the VP was raised; there must now be a VP review to ensure all aspects of the final approved URS are authorized and planned for.
With an approved VP in place, a start can be made in authoring, reviewing and publishing the pharmaceutical equipment validation qualification protocols that are required to verify that all the requirements documented in the URS and all applicable cGMP requirements are complied with. These are:
- Design Qualification (DQ).
- Installation Qualification (IQ).
- Operational Qualification (OQ).
- Performance Qualification (P1Q).
- Process Qualification (P2Q).
Where vendor developed Functional Specifications (FS) and or Design Specification (DS), are available they should be reviewed and referenced in the VP. Where these documents are not available a DS or FS may have to be retrospectively developed. The Design Qualification (DQ) must be used to verify that this FS or DS (if proceeded with) will deliver a system compliant with all requirements in the URS and the applicable cGMP rules and regulations. The execution of this DQ must verify that the proposed design will;
- Perform as specified in the URS.
- Conform to all mandated cGMP requirements.
- Operate in a manner safe to the product, and the operations staff.
The installation of each piece of pharmaceutical validation equipment must be subjected to, and satisfy, a pre-approved Installation Qualification (IQ) protocol. When the requirements of the IQ have been satisfied, all aspects of the operational capabilities of each system must be fully challenged and verified by the execution of a pre-approved Operational Qualification (OQ) protocol. As soon as the executed IQ and OQ protocols having been reviewed and approved, a pre-approved Performance Qualification (P1Q) protocol or Process Qualification (P2Q) (this requirement will be documented in the VP) must be issued for execution. The execution of this PQ must verify that the system performance requirements, as specified in the URS have been achieved, and that the system operates in a manner safe to the product and production personnel.
All validation and qualification relates to a regulated product either directly or indirectly.
Direct pharmaceutical equipment validation; refers to the validation and qualification of all equipment that is actually used in the manufacture of the the product (mixers/ovens/autoclaves etc.)
Indirect pharmaceutical equipment validation; refers to the validation and qualification of all equipment that must be in place to support the direct equipment and or is required to deliver any specific environmental conditions specified in a process in use. (process air/water/HVAC/isolation etc).
Pharmaceutical Equipment Validation Implementation.
You have a new product and you are tasked with the pharmaceutical equipment validation for the entire project. Along with the facilities and utilities, being put into place to manufacture this product.
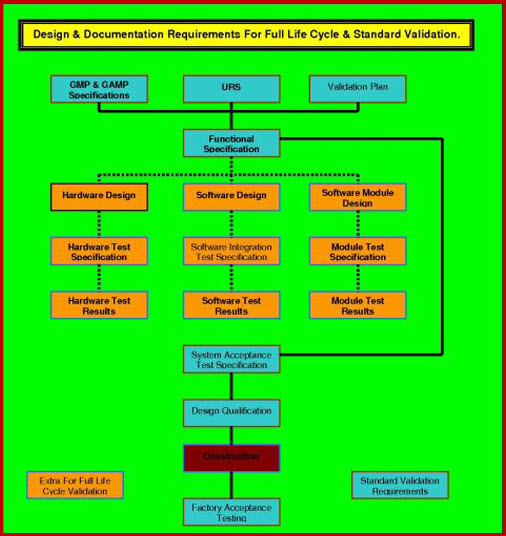
Diagram 1. Full Life Cycle Validation (FLCV).
Task 1. Validation Plan
Validation Master Plan (VMP) gives an overall depiction of the company facilities, along with the management structure, and details of how cGMP is, or is to be, integrated with all company activities. On the other hand the pharmaceutical Equipment Validation Plan (VP) is used to manage pharmaceutical equipment validation and qualification projects that are smaller in size and have easily defined boundaries.
Raise the appropriate Validation (Master) Plan (VMP/VP) as described in https://www.validation-online.net/validation-master-plan.html or purchase, and download and edit one from "http://quality.validation-online.net/validation-documentation.html" Complete all requirements and circulate draft copy of document for peer review.
Incorporate all circulation comments and submit for approval.
Issue document for project management
use. This comprises document One of the set of Eight required for Pharmaceutical Equipment Validation.
Task 2. Introduction to Matrix.
Raise a Validation and qualification Documentation Matrix (10000002) chart as described in https://www.validation-online.net/validation-documentation-matrix.html or purchase and download one from "http://quality.validation-online.net/validation-documentation.html" and in the ‘description’ column, list all equipment that requires to be reviewed for validation assessment.
As far as possible where pharmaceutical equipment validation operates as a system, it should be listed as a system. Where it does not, or can not, then list individual equipment. For each listing raise individual User Requirement Specifications. Either author one in accordance with https://www.validation-online.net/user-requirements-specification.html or purchase and download one from OUR DOCUMENT SHOP.
Issue document for project management use. This comprises document number Two of the set of Eight required for Pharmaceutical Equipment Validation.
Right click in each cell of the spreadsheet, and open a Comments page. Insert into the comments page;
Person responsible for the document:
Date started:
Date first review:
Date final review:
Date issued:
Date executed:
Date execution reviewed & accepted.
The above data would normally be monitored and controlled from a planning schedule (such as Microsoft Project as shown on Diagram 3), however it is important to have this matrix in place as the master record of all project details.
Diagram 3. Plan.
Task 3. Matrix Population.
As items are added to the matrix, a unique number is allocated to each one, in Column 1. This is the pharmaceutical equipment validation number, individual protocols are subsequently identified by adding the document acronym to the end of the unique number. This way all validation documents for an item have the same identifying number. Progressively subject each item listed in the matrix, to the questions in the Validation Risk Assessment (VRA), which should either be authored in accordance with https://www.validation-online.net/validation-risk-assessment.html or purchased and downloaded from http://quality.validation-online.net/ .
As each piece of pharmaceutical equipment validation completes the VRA and is given a risk rating, and an assessment for part 11 compliance, enter these ratings into column seven of the matrix, in the row allocated for the equipment. Format, (risk rating)(Y or N for part 11 compliance).
Issue document for project management use. This comprises document Three of the set of Eight required for Pharmaceutical Equipment Validation.
Note:
Risk Assessment (RA) in the pharmaceutical / biotech / medical device validation industry, is often misunderstood. In regulated industries RA’s are used for a many different purposes. In our case we are only considering the use of an RA to justify the depth and scope of our validation and qualification requirements. As such the VRA has to simply ascertain; what scope of validation this system / pharmaceutical equipment qualification must be subjected to, to ensure it is correctly validated, and to ascertain whether the system has to conform to 21 CFR Part 11.
Task 4. User Requirement Specification (Sections 1) (URS).
For each system/item listed in the matrix raise a user requirements
specification as (URS) as described in https://www.validation-online.net/user-requirements-specification.html
or purchase and download one from http://quality.validation-online.net. Register each URS by giving it a
unique number and entering that number into the matrix column.
Circulate the registered URS templates to the individual equipment owners as specified in the project VP/VMP. Request owners complete section one of URS. Issue document for project management use. This comprises document Four of the set of Eight required for Pharmaceutical Equipment Validation.
Task 5. User Requirement Specification (approval), (URS).
On complete of Section One requirements, the URS must be reviewed and approved. The approved URS must then be forwarded to the procurement team. It is the procurement team’s responsibility to ensure that each individual invitation to tender, has the appropriate URS attached. Issue document for project management use. This comprises document Five of the set of Eight required for Pharmaceutical Equipment Validation.
Task 6. Design Qualification (DQ).
Ensure all preceding documents are signed off and approved.
When all vendor (and in house) design proposals have been submitted, (received from in, or out of house sources) the accepted design, must be subjected to a DQ, to validate it is fit for purpose. A Design Qualification (DQ) protocol must be raised
in accordance with https://www.validation-online.net/design-qualification.html or purchase and download one from http://quality.validation-online.net .
The approved DQ must be executed to validate that the design is robust and has been subjected to sufficient proof of concept testing, to establish that if proceeded with, it will satisfy the requirements listed in the URS. Issue document for project management use. This comprises document Six of the set of Eight required for Pharmaceutical Equipment Validation.
Task 7. User Requirement Specification (Sections 2 ) (URS).
The URS top level functionality is further broken down into sub-functions in the Design Specification (DS). The vendor must therefore complete Section 2, of the URS, documenting the relationship between the URS functionality and the actual design functionality. This is required to enable compliance with the requirement for maintaining the traceabilty from URS to software code, as further described in Task 9. Issue document for project management use. This comprises document Seven of the set of Eight required for Pharmaceutical Equipment Validation.
Task 8. Installation Qualification
Raise an Installation Qualification as described in https://www.validation-online.net/installation-qualification.html or purchase and download one from http://quality.validation-online.net/ Complete all requirements and circulates draft copy of document for peer review.
Incorporate all review comments and submit for approval.
Ensure all preceding documents are signed off and approved, prior to executing this document.
Issue document for execution.
Review executed document.
Produce summary report.
Issue document for project management use. This comprises document Eight of the set of Eight required for Pharmaceutical Equipment Validation.
Task 9. Operational Qualification.
Raise an Operational Qualification as described in https://www.validation-online.net/operational-qualification.html, or purchase and download one from http://quality.validation-online.net/ Complete all requirements and circulates draft copy of document for peer review.
Incorporate all review comments and submit for approval.
Ensure all preceding documents are signed off and approved, prior to executing this document.
Issue document for execution.
Review executed document.
Produce summary report.
Issue document for project management use. This comprises document Nine of the set of Eight required for Pharmaceutical Equipment Validation.
Task 10. Performance Qualification.
Raise an Performance Qualification as described in https://www.validation-online.net/performance-qualification.html, or purchase and download one from http://quality.validation-online.net/. Complete all requirements and circulates draft copy of document for peer review.
Incorporate all review comments and submit for approval.
Ensure all preceding documents are signed off and approved, prior to executing this document.
Issue document for execution.
Review executed document.
Produce summary report.
Issue document for project management use. This comprises document Ten of the set of Eight required for Pharmaceutical Equipment Validation.
News for Today
User Requirements Specification Template
$115.00
The User Requirements Specification template is the document that sets the standard, and specifies your requirements in a manner that ensures when a system or piece of equipment is selected for cGMP use that all essential support elements; i.e. maintenance, parts, operator & maintenance training, are planned and budgeted for. It uses three levels of User Requirements Specification Template (URS), URS Level 1, 2 and 3, and is the only URS to guarantee traceability from the URS through to the final OQ and PQ functionality testing. A requirement mandated by cGMP regulations. It can be used on mechanical, electrical and software controlled, monitored or driven systems.
Corporate Validation Manual $1,160.00
This definitive 1000 + page (including all attachments) Corporate Validation Manual arrives with you in USB memory stick format, this enables you at any time to download protocol or test-scrips documents and quickly edit them into company bespoke documents. In fact there are over $3,500.00 worth of superb documents, that form attachments to the DVM manual, which can be instantly copied. Once copied, the unique document interactive editing, allows you to produce high quality bespoke company documents; Such as the Validation Plan Template or the equally ubiquitous User requirements Specification template Design Qualification (DQ); in a few hours. The cost of the Definitive Validation Manual, will be recouped in the first few weeks of use. It will then go on to show a massive return on your original investment.