VALIDATION QUALITY PLAN
"A validation quality plan must define, detail and mandate means and methods by which an approved change control concept can be progressed through the regulatory qualification process and into cGMP compliant production"
Validation Quality Plan.
The Validation Quality Plan (VQP) is probably the most important of
all documents in a validation project. It is an essential document in giving
assurance that the validation task can be successfully executed in an effective, timely
and cost effective manner. Simply because
this is the stage in the operation where all aspects of the validation are
highlighted, debated, allotted and budgeted for. Once the full scope of the
validation task is unearthed; then and then alone, can all these facets be
catered for and sensible time lines and responsibilities deliberated. To this end there may be several VA in
any major validation project. Each VP will cover
identical or similar equipment where the validation responsibilities and scopes
are identical or similar and can be adequately defined in one validation quality plan.
The top level of the User
Requirements Specifications (URS) must be in place to enable
the author(s) of the VP to start assessing; not only the basic user level
requirements but the attendant needs that the user level requirements make
requisite, such as; staff levels, user training, maintenance training, utility
and facility loadings. Along with
these requirements will come a multitude of other minor and major issues that
only ever become obvious after such extended and invasive studies are
completed. Only when these
requirements are fully reviewed and documented, can they be accurately
quantified. However once they are
defined, then the VP can start to allocate and document individual staff
responsibilities and individual and collective achievable time lines. Now the stage has arrived where a defined,
detailed and workable validation quality plan can be documented, reviewed, and subject
to reviewed comments being incorporated; issued by management as the official Validation
Quality plan of action.
The VP is the document that the company must use to authorize actual validation
methodologies, scopes and associated individual responsibilities (by job
title). It therefore has to work hand in hand with the
Validation
Risk assessment (VRA). The VA must list all the equipment that
it applies to and the VRA must be executed against each of the items in this
list. On completion of each
execution the appropriate scope of validation will be indicated by the VRA. This scope must be entered into the
appropriate section of the VP, before it becomes an approved and locked
controlled document. The VP will
therefore list the scope of validation applicable to each listed item along with
any individual specific methodologies that should be used.
Validation Quality Plan Scope.
The Validation Master Plan (VMP) is the single most important document because it describes the basic concept for your overall site validation program. This interactive detailed document is a delight and simple to use. The generic template and attached SOP lead you through the whole process quickly and seamlessly. This thirty page document is suitable for all types of pharmaceutical /bio-technical / medical device / API, manufacturer and or processor. The VMP addresses process validation, facility validation, utility validation, equipment qualification and cleaning validation. The objective is to define responsibilities, outline your methods involved in the qualification and validation of your facility, define the areas and systems to be qualified and validated and to provide a program for achieving and maintaining a validated status.
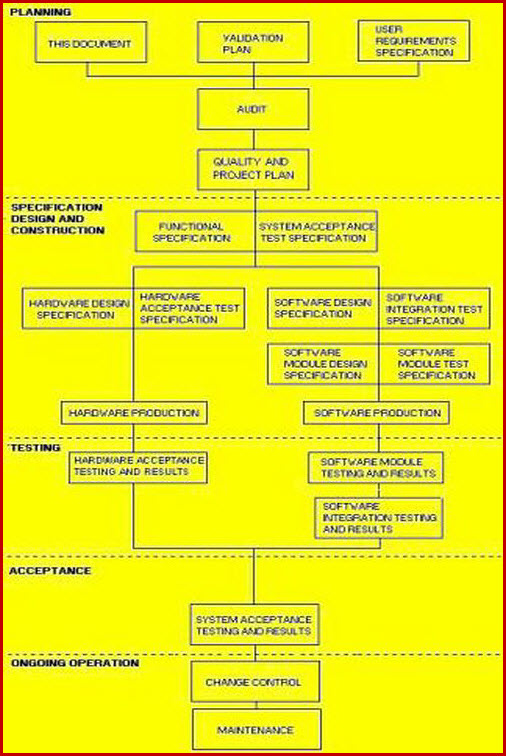
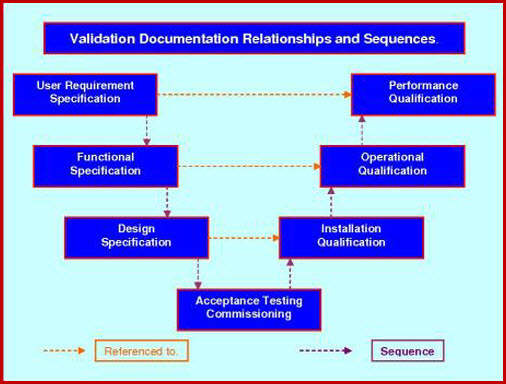
The VP Role In The Validation Process.
Validation Quality Plan Progress.
21 Years of retailing cGMP - 35k templates supplied to the industry.
User Requirements Specification Template
$115.00
The User Requirements Specification template is the document that sets the standard, and specifies your requirements in a manner that ensures when a system or piece of equipment is selected for cGMP use that all essential support elements; i.e. maintenance, parts, operator & maintenance training, are planned and budgeted for. It uses three levels of User Requirements Specification Template (URS), URS Level 1, 2 and 3, and is the only URS to guarantee traceability from the URS through to the final OQ and PQ functionality testing. A requirement mandated by cGMP regulations. It can be used on mechanical, electrical and software controlled, monitored or driven systems.
Corporate Validation Manual $1,160.00
This definitive 1000 + page (including all attachments) Corporate Validation Manual arrives with you in USB memory stick format, this enables you at any time to download protocol or test-scrips documents and quickly edit them into company bespoke documents. In fact there are over $3,500.00 worth of superb documents, that form attachments to the DVM manual, which can be instantly copied. Once copied, the unique document interactive editing, allows you to produce high quality bespoke company documents; Such as the Validation Plan Template or the equally ubiquitous User requirements Specification template Design Qualification (DQ); in a few hours. The cost of the Definitive Validation Manual, will be recouped in the first few weeks of use. It will then go on to show a massive return on your original investment.