VALIDATION TEMPLATES
Since this is the era of "Risk Based" software validation - it is of the utmost importance to ensure that the validation scope is risk derived. Consequently a purposed designed Validation Risk Assessment (VRA) is absolutely essential if flawless validation is to be achieved.
Validation Templates Innovation.
Validation Templates design is of critical importance when regulatory compliance and clarity of purpose are prerequisites. In these circumstances it is only the bold and confident that will actually strike out using innovative and intuitive thinking to produce templates that can actually save in time and cost.
It is the desire to reduce costs that has led to the introduction of our 4Q template design, where It is left to the 4Q protocol to apply all the verification tests and inspections.
So what does the VrrP actually achieve. It leads (with built-in prompts) a competent person through the process of defining; the validation task boundaries, identifying and allocating all responsibilities, protocol contents and methodologies, application of risk mitigation, Structure of test scripts, Sequencing of test scripts execution.
4QTM Equipment Validation Protocol.
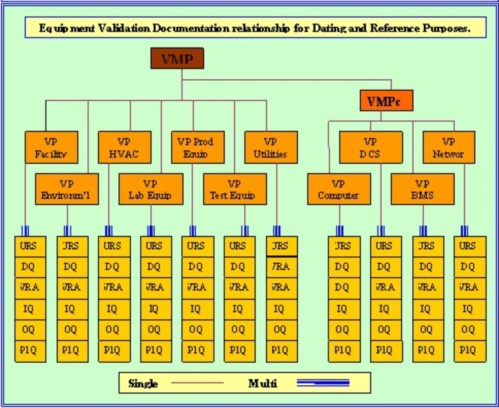
This new dynamic 4Q Equipment Validation Protocol (4Q-Equip) has been designed specifically to replace four standard protocols. By taking the contents of the these four protocols and carefully weaving them into one notably easy to use protocol, we have made significant progress in reducing validation paperwork. Reductions of up to 75% have been quoted as the likely total. Integrating the old style DQ/IQ/OQ/PQ protocols into one 4Q document will be an enormous savings in man hours in the authoring, reviewing, updating and approving tasks. With the simultaneous introduction of the new Validation risk & Requirements Plan (VrrP) which integrates the VP, VRA & URS into one document - equipment validation has been reduced to two document.
The User Requirements Specification (URS) is raised by the end user at URS Level One stage, and specifies what the end user requires the equipment to do. Additionally, any regulatory guidance, mandatory instructions or conditions of use, must be included into the URS. The URS must be the subject of one or more peer reviews where the user, the maintainer and QA, review and ensure that the document is clear and concise in all the requirements detailed.
Design Qualification is used at the stage where a design that has been developed from the, VMP / URS /GAMP 5 / cGMP / and other Health and Safety Guidelines, is reviewed and commented on by competent persons to ensure that the designed equipment, if built, will satisfy all the detailed specified requirements. It can also be used to review an of the shelf item to ensure it will satisfy the URS.
The Installation Qualification (IQ) must verify that the equipment has been installed, activated or put into use in accordance with the manufacturer’s requirements, cGMP rules and regulations, and all applicable health and safety requirements. The IQ should further include a review of maintenance procedures, repair parts lists, and calibration methods
The execution of the Operational Qualification (OQ) protocol verifies that the functionality of the equipment conforms with functionality specified in the Functional Specification (FS) for the system. This verification is achieved by subjecting the equipment to a range of fully documented inspections and tests.
Validation Templates Documentation Interface.
Authoring Quality Protocols.
It is imperative that all tests and inspections detailed in the validation template for VMP and other protocols are clearly and concisely detailed. Each test and or inspection must be justified by quoting the requirement that mandates it. Validation scopes, boundaries and responsibilities must be set out in the Validation Plan (VP). Protocol authors should never be allowed to commence construction of validation template protocols without being made aware of the company constraints, definitions, scopes, methodology and individual responsibilities as authorized by the company practice and procedure policies as promulgated by the company. For precise effective validation all protocol tests and inspections must be cross-referenced to the requirements detailed in the User Requirements specification, which mandates their inclusion.
Over-all protocol standards are detailed in the SOP’s for the different protocols and are incorporated into template for VMP along with all our other templates. Here we are concerned about the testing element alone. All testing must be detailed and pre-approved by a qualified person to ensure the system under test has been adequately tested. Each test must must be justified and verified as fit for purpose comprise of;
Main Sub-headings in Test Scripts.
Rationale; giving the reason and or object of the test.
Test Method; giving details of the desired test methodology.
Acceptance Criteria; giving details of the anticipated acceptable results of the tests.
Test Result; giving details of the actual results obtained that satisfied the acceptance criteria.
General details that must be adhered to.
The test result must be initialled (or signed) by the person executing the tests, on completion or at each significant stage.
Each test must be designed to verify an element of the equipment functionality.
Each test must a have a result that is clear, unambiguous and known.
The test method must call up for the recording of the test result parameters. (No ticks or tick boxes, no generalities).
Each test must be witnessed or the results must be reviewed by a competent person.
The overall test results must be approved by a competent person.
21 Years of retailing cGMP - 35k templates supplied to the industry.
Combined IQ/OQ/PQ for Spreadsheets. (issue-2) $159.00
This validation online combination protocol has been specifically designed to verify that all aspects of your spreadsheet conform to best practice and that the spreadsheet layout ensures consistent and accurate use and results. The tests and inspections normally authored in separate protocols have been assembled in one protocol which is divided into three sections. This protocol enables you to verify that your developed spreadsheet application is GMP compliant, thus avoiding 483s and warning letters. You can now validate your application with minimal documentation. Equipment Validation Protocol, validation online protocol template.