How How to prepare GMP Compliant Quality Plans
How How to prepare GMP Compliant Quality Plans
Understanding how to prepare GMP Compliant Quality Plans must start with Primary Requirements (PR). However these PR’s inevitably will have Support Requirements (SR) and further along the line Ancillary Requirements (AR). It is essential that all these requirements are identified as early in the procurement stage as is possible. Failure to identify and consequently not provision for; SR’s and ER’s, has and still is one of the main causes of validation program delays and cost overruns. We always recommend that companies involved in URS authoring develop their own exhaustive check list of all possible SR’s and AR’s. This check list can then be used by all persons involved in authoring FDA Quality Plan and User Requirements Specification documents; as an infallible aid de memoir.
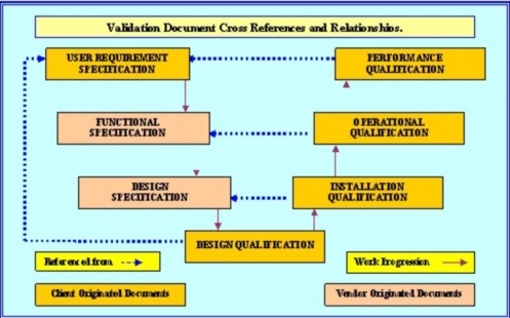
The Validation task ends with the PQ; which is required to verify that all the requirements as detailed in the URS have been challenged, inspected and or tested in accordance with and results obtained confirm complete compliance with their respective documented acceptance criteria.
(i.e. no URS; equates to no content for your PQ = flawed validation).
In between are the FDA Quality Plan (Validation Plan) (VP or QP),
Validation Risk Assessment (VRA) and the Design Qualification (DQ). The regulatory authorities require these documents to be in place with the dates for content approval and execution of running consecutively.
The devil is in the detail: The User Requirements Specification (URS) must be fully detailed and include all aspects of procurement; such as usage, maintainability, calibration, cleaning, utility requirements, facility requirements, user training, support documentation, support engineering drawings, software programs and all the attendant SOP’s that are required in the execution of the these and all other GMP specified tasks. There must be no surprises or shocks when the machine / software arrives, all requirements (yes even the most obscure details) must be anticipated and engineered for.
If you tackle the URS in a professional manor with your colleague’s and peers, you should develop a sound company reviewed and approved document that will guide, develop and produce a comprehensive User Requirements Specification (URS); that will prove to be worth its weight in gold.
The FDA Quality Plan
How to prepare dynamic GMP Quality Plans stated that over 70% of validation problems stem from the procurement not being properly detailed and or managed. Get the URS correct and you are well set to execute your validation task smoothly, compliantly, on time and on cost. The execution of your VRA defines and authorizes your whole approach to the validation of any software used in the or system undergoing qualification. Software used in this type of equipment can normally be validated by testing its functionality against the requirements documented in the URS document along with verification that the software was developed using an approved FDA quality plan appropriate for the GMP requirements for this class of software.
It must be getting rather obvious now that the documentation required for compliant validation does indeed form a chain, with each link firmly supported by the previous link and forming a sound footing for the next link in the progress towards qualification.
The FDA Quality Plan is defined in the manual which arrives with you in DVD format, complete with a search facility that enables you at any time to have the benefit of over thirty years of validation experience and wisdom, right beside you. Showing you How to prepare GMP Compliant Quality Plans cGMP Quality Plan access references, instantly down load test scripts, and above all, have access to the best FDA Quality Plan document templates available anywhere.
If you have background knowledge in pharmaceuticals and are reasonably competent, the Definitive Validation Requirements Manual will do the rest for you by detailing all validation requirements. If you are in the Pharmaceutical / Biotechnological / API / Medical Device, industries or whether your in any of the engineering streams that are involved with these industries, the Definitive Validation Manual will enable you to:- • Get started in pharmaceutical validation. • Gain promotion in the industry. • Become dual skilled. • Get into the highest paid section of the industry. The cost of the FDA Compliant, Definitive Validation Requirements Manual, will be recouped in the first few weeks, it will then go on to show a massive return on your original investment. An investment that you will never ever regret.
June 2015, The FDA is to Create a New Drug Quality Office: The agency said the new office will publish a 'dashboard' of quality findings that will show the range of metrics, good and bad, achieved by the industry.........In September 2003, The Wall Street Journal published an article informing all that pharmaceutical “manufacturing techniques lag behind those of potato-chip and laundry-soap makers.” The same article correlated the rise in recalls with FDA quality plan problems and noted that despite fines in excess of US$500 million for manufacturing failures, acceptable levels of attaining how to prepare dynamic GMP Quality Plans are not being achieved.
FDA Quality Plan Graphics.
21 Years of retailing cGMP - 35k templates supplied to the industry.
Combined IQ/OQ/PQ for Spreadsheets. (issue-2) $159.00
This validation online combination protocol has been specifically designed to verify that all aspects of your spreadsheet conform to best practice and that the spreadsheet layout ensures consistent and accurate use and results. The tests and inspections normally authored in separate protocols have been assembled in one protocol which is divided into three sections. This protocol enables you to verify that your developed spreadsheet application is GMP compliant, thus avoiding 483s and warning letters. You can now validate your application with minimal documentation. Equipment Validation Protocol, validation online protocol template.