How to write a superb Machinery Validation protocol.
Introduction to Machinery Validation.
Understanding how to write a superb machinery Validation protocol is fully explained in the integral SOP contained within each template. Machinery validation or Qualification is a mandatory requirement and must be applied to all equipment whenever it is used in a way that can affect the quality, safety, efficacy of a controlled product or jeopardize the integrity of any predicated data, relating to that product. The term machinery relates to any metal moving or removing equipment which is largely mechanical in operation, but may have software management or recording facilities. Examples; could be metal working machinery such as CNC - machining-centers - milling - turning - drilling - packaging machinery.
All equipment that executes any process that can or could change, damage or obscure the quality, efficacy or manufacturing records of a regulatory controlled product, must be qualified by being validated.
In the US this is managed by the FDA. In Europe it is managed by EU. Many other countries also have an agency to manage this requirement and those that don’t usually rely on the World Health Organization (WHO). Understanding how to write a superb machinery validation protocol comes down to the authoring experience of each author.
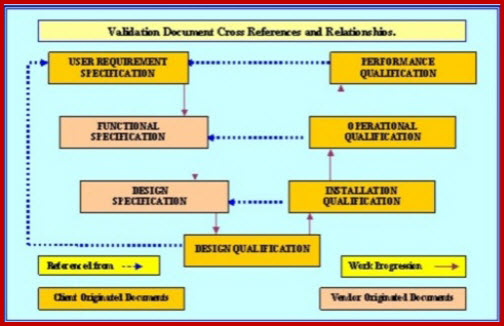
Machinery Validation and or qualification requires a suite of inter-related documents to be put into place. Each document should be constructed to a format and with a content that will satisfy the regulatory expectation for such a document. The regulations leave a certain amount of freedom to manipulate tests and inspections to save unnecessary retesting and ease access and downtime requirements. However this freedom does not affect the basic directives; which mandates, the documented need for the suite of documents to start at the VP then the URS and on through the VRA, DQ, IQ, OQ to the PQ. These regulatory requirements exists and must be complied with.
Click here to view - How to write a superb Machinery Validation Protocol.
Machinery Validation Practices and Procedures.
Understanding how to write superb machinery validation protocols is normally started with the submission of a requirement or group of requirements. For existing facilities this should take the form of a Change Request (CR). As soon as management has agreed to proceed with the CR, approval should be issued to produce a Validation Plan; sometimes called Quality Audit Plan (VP). This plan must be all encompassing. It must give assurance that all aspects of the proposed CR have been studied and the CR impact on existing facilities, utilities, product and personnel have been defined and the appropriate corrective or support actions planned for. A fully detailed User Requirements Specification (URS) can now be authored reviewed and published. Since developing the URS may raise problems that could not be anticipated when the VP was raised; a VP review is required to ensure all aspects of the final approved URS are fully catered for.
With the URS defined and the Validation Plan (VP) in development Validation Risk Assessment (VRA) must be authored and executed to establish the scope and depth of CNC validation that is appropriate for this equipment. This information must be published in the VP and used as the authority for all protocol development. Where vendor developed Functional Specifications FS) and or Design Specification (DS), are available they should be reviewed and referenced in the VP. Where these documents are not available a DS or FS may have to be retrospectively developed.
When the DS or FS that is to be used are defined, a pre-approved Design Qualification (DQ). The execution of this DQ must verify that the proposed design will;
Operate in a manner safe to the product, and the operations staff.
The installation of each validatable item and or system must be subjected to, and satisfy, an approved Installation Qualification (IQ) protocol. Details of the scope of the IQ, responsibilities for generating, reviewing and approving of this document must all be documented in the VP.
When the requirements of the IQ have been satisfied, all aspects of the operational capabilities of each system must be fully challenged and verified by the execution of a pre-approved Operational Qualification (OQ) protocol. As with the IQ; OQ scope and details of the persons responsible for generating, reviewing and approving of this document will be documented in the VP.
As soon as the executed IQ and OQ protocols having been reviewed and approved, a pre-approved Performance Qualification (P1Q) protocol or Process Qualification P2Q) (this requirement will be documented in the VP) must be issued for execution.The execution of this PQ must verify that the system performance requirements, as specified in the URS have been achieved, and that the system operates in a manner safe to the product and production personnel. All authors must demonstrate that they know how to write a superb machinery validation protocol.
MACHINERY VALIDATION or QUALIFICATION
21 Years of retailing cGMP - 35k templates supplied to the industry.
SOP for Spreadsheet Creation. $125.00
Why does something as simple as a spreadsheet figure in so many regulatory citations? Good question; and at times a difficult one to answer. When you ask a group of compliance personnel the same question you will be informed that Excel cannot be validated because it does not seal the original copy (of the spreadsheet), allows the original to be modified and has an audit trail that can be disabled. All true, but none of these problems interfere with your ability to validate that the spreadsheet is fit for purpose. They only preclude you from using the spread sheet as a compliant repository for any data that has to be store in compliance with 21 CFR Part 11.
If the spreadsheet is signed off and dated by the user, their supervisor and QA, it becomes regulatory acceptable data stored in hardcopy, and Part 11 does not apply.
After numerous requests for this, we have launched our brand new SOP for Spreadsheet Creation to cover these and other known target points that the regulators consistently hone into as soon as they find that spreadsheets are being used. Use this Spreadsheet Creation SOP to ensure that you create spreadsheets that are validatable. Then use our spreadsheet validation pack to validate them.
Equipment Combined IQ/OQ/PQ Protocol (Issue-2). -- $159.00
This combination protocol has been produced in response to several hundred reader suggestions we received in our ‘Suggestions Section’. It has been carefully designed to make it the preferred choice for Process and Laboratory stand alone equipment. It is interactive, easy to use and suitable for all mixes of equipment with and without software.<br>
The IQ section establishes documented verification that key aspects of the equipment adhere to approved design intentions and that the recommendations of the manufacturer have been suitably considered. The OQ section establishes that there is documented verification that the installed system functions as specified and that there is sufficient documentary evidence to demonstrate this. The PQ section gives documented verification that the equipment performance in its normal operating environment is consistently exactly as specified in the URS